
Erdogan Dreams of an Ottoman Grandeur
By BENNY AVNI
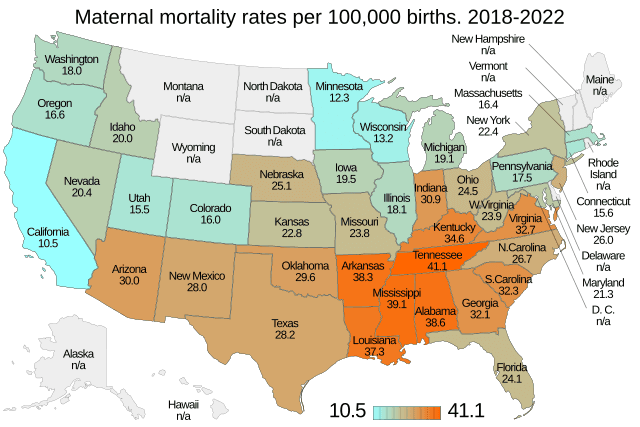
|Such warnings are reserved for medications that carry risks of death or life-threatening reactions.

Already have a subscription? Sign in to continue reading
$0.01/day for 60 days
Cancel anytime
By continuing you agree to our Privacy Policy and Terms of Service.

By BENNY AVNI
|
By THE NEW YORK SUN
|
By JOSEPH CURL
|
By HOLLIE McKAY
|
By HOLLIE McKAY
|
By MATTHEW RICE
|
By GEORGE WILLIS
|
By THE NEW YORK SUN
|