
Love and Resilience Derived From ‘Monsters’ and Martial Arts
By ELYSA GARDNER
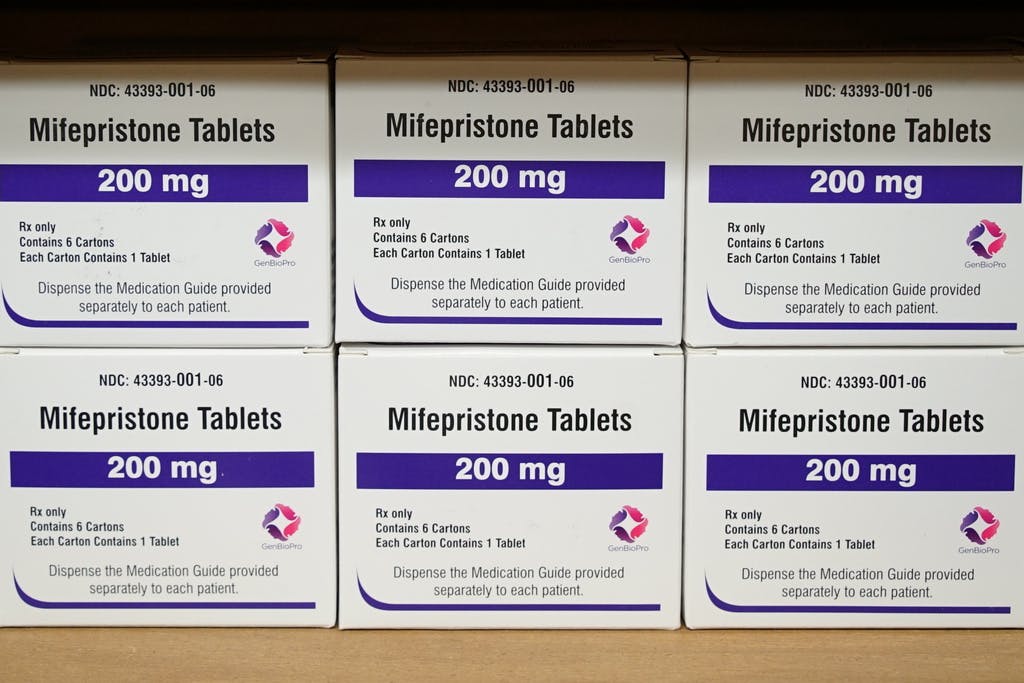
|The ruling, which is likely to be taken to the Supreme Court, vindicates, for now, the 1873 Comstock Act that banned using the mails for abortion materials.

By ELYSA GARDNER
|
By ELYSA GARDNER
|
By NOVI ZHUKOVSKY
|
By LUKE FUNK
|
By LAWRENCE KUDLOW
|
By JOSEPH CURL
|
By THE NEW YORK SUN
|
By BRADLEY CORTRIGHT
|Already have a subscription? Sign in to continue reading
$0.01/day for 60 days
Cancel anytime
By continuing you agree to our Privacy Policy and Terms of Service.